Improving the Detection of Intestinal Parasites Using Centrifugal Flotation Techniques
By Joseph Mueller, Dane County Humane Society Medical Services Intern

Intestinal parasites are common causative agents of disease in shelter medicine as well as small animal private practice. These diseases can be acute, chronic, or subclinical and can produce a variety of symptoms, including diarrhea, vomiting, weight loss, and anemia. The most important aspect of a proper diagnosis of most intestinal parasites is recovering and identifying parasite eggs, cysts, or oocysts from fecal samples. Fecal flotation is the standard diagnostic screening technique for most of these parasite structures. This method relies on the differences in the specific gravity of the eggs, fecal debris, and floatation solution. A solution with high specific gravity will concentrate the lower-gravity eggs at its surface and allow for a clean preparation for microscopic examination with minimal fecal debris present.
There are many different procedures and flotation solutions used in veterinary medical practice. Simple or passive flotation is a quick and easy procedure that is often employed due to a lack of resources, time, or familiarity with alternative techniques. Centrifugal flotation, however, is a more sophisticated method and has been recommended by the Companion Animal Parasite Council as a “Best Practice” for all fecal examinations. Multiple studies have demonstrated that centrifugal flotation techniques consistently recover more parasite eggs from small animal feces. By spinning the floatation preparation in a centrifuge (example right), a greater force is placed on the heavier fecal debris, forcing it to the bottom of the centrifuge tube much quicker than gravity would by itself.
There are many different procedures and flotation solutions used in veterinary medical practice. Simple or passive flotation is a quick and easy procedure that is often employed due to a lack of resources, time, or familiarity with alternative techniques. Centrifugal flotation, however, is a more sophisticated method and has been recommended by the Companion Animal Parasite Council as a “Best Practice” for all fecal examinations. Multiple studies have demonstrated that centrifugal flotation techniques consistently recover more parasite eggs from small animal feces. By spinning the floatation preparation in a centrifuge (example right), a greater force is placed on the heavier fecal debris, forcing it to the bottom of the centrifuge tube much quicker than gravity would by itself.
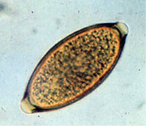
The lighter parasite eggs are driven to the top of the meniscus and the elimination of the heavier fecal debris from the surface provides a cleaner preparation with less material to obscure the view of the parasite eggs. Thus, more eggs are recovered and the examiner is more likely to see the eggs that are present on the slide. This higher sensitivity would be especially important in diagnosing chronic parasite infections that are often characterized by small parasite numbers and infections by parasites that tend to produce low numbers of eggs. In addition, when using a viscous solution like Sheather’s Sucrose, the downward force created by the centrifugation enhances the buoyancy of the eggs and helps them overcome the force of viscosity on their way to the surface. The advantage of using centrifugal flotation is particularly important for detection of canine whipworm infections by Trichuris vulpis (image left), whose eggs are quite dense and float less readily.
The following procedure is taught at the University of Wisconsin-Madison School of Veterinary Medicine and is representative of centrifugal flotation techniques.
The protocol implemented at each shelter or clinic can certainly be modified to fit their needs and circumstances and can likely be streamlined from the above process with comparable results achieved. For example, it may not be completely necessary to initially mix and centrifuge the feces in water.
References
- Mix 2-4 grams of feces in approximately 10-12 mL of water.
- Strain through a tea strainer into a clean cup.
- Add the filtrate to a centrifuge tube, counterbalance the tube, and spin at 2000 rpm (setting “5 or 6”on a clinical centrifuge) for 5 minutes.
- Pour off the supernatant. Invert the tube on paper towel and let drain for 1 minute. Resuspend the pellet in the flotation solution.
- Counterbalance and spin at a setting of “2” for 10 minutes.
- Transfer several drops to a microscope slide by touching a clean wire loop to the surface of the fluid.
- Cover slip and examine under 10 X objective.
The protocol implemented at each shelter or clinic can certainly be modified to fit their needs and circumstances and can likely be streamlined from the above process with comparable results achieved. For example, it may not be completely necessary to initially mix and centrifuge the feces in water.
References
- Dryden MW, Payne PA, Ridley R, et al. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther 2005; 6: 15-28.
- Katagiri S, Oliveira-Sequeira TC. Comparison of three concentration methods for the recovery of canine intestinal parasites from stool samples. Exp Parasitol 2010; 126(2): 214-6.
- Zajac A, Johnson J, King S. Evaluation of the importance of centrifugation as a component of zinc sulfate fecal flotation examinations. J Am Anim Hosp Assoc 2002; 38: 221-224.
